 Open Nav
Open Nav
 Open Nav
Open Nav
Targeting future energy devices, we are pursuing fundamental, practical investigations for development of materials and their interfaces for electrochemical energy devices such as rechargeable batteries and fuel cells. As device performance is strongly influenced by electrochemical interfaces as well as the materials in an energy device, we are studying electrochemical formation of materials, interfaces, and measurement methods including AC techniques and analysis procedures.
To realize advanced electrochemical power supply for portable equipment, some issues must be taken into account, i.e., 1) highly effective electrochemical reactions, 2) lower energy loss due to internal resistance, 3)longer life time, 4) guaranteed safety and 5) lower production costs. Secondary batteries with high power output and high capacity is one of the targets. Fuel cells are another target for improvement for future energy supply. Secondary batteries using Li metal anodes are in strong demand as possible future batteries with high energy density. It is necessary to improve or develop the materials used in the metallic Li anode batteries to ensure safety during their operation. We are investigating the interfaces between metallic Li and electrolytes with the aim of modifying them. We are also introducing solid polymer electrolytes to suppress dendritic growth of Li while the battery is being charged. Some Li-alloy anodes are also investigated to secure safety and a longer life than with conventional anode materials. Research on Li or Li alloy anodes requires characterization of the interface, where charge transfer reactions take place. At the interface, a so-called SEI (solid electrolyte interface) layer is formed by the reaction caused by contact between anodes and electrolytes. The performance of anodes depends heavily on the characteristics of the SEI. The SEI should be permeable for Li+ ions and prevent further sub-reactions between the anode material and the electrolyte components. We are conducting elemental analysis and electrochemical characterization of SEIs to acquire understanding of the nature of SEIs for designing of SEIs for anodes in future Li batteries.
The three-phase boundary of fuel cells, where the reaction generates the electric energy, is a sophisticated interface. We are characterizing the structure of the three-phase boundary as well as the transportation pathways of electrons, ions and gases by electrochemical methods. The mechanism of the degradation of fuel cell performance is being discussed along with the electrochemical responses for further improvement of fuel cells.
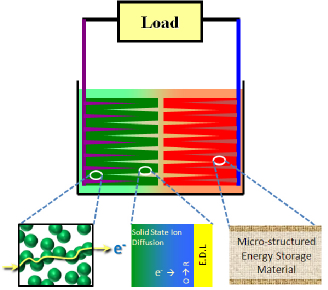
Elementary processes of the electrochemical energy device. All the factors to improve the device properties are investigated and the materials and the structure are designed.
B. S. Waseda University (1990); Dr. Engineering, Waseda University (1995); Research Associate, Waseda University (1995); Post-doctoral Fellow, University of Minnesota (1996-1997); Lecturer Waseda University(1997-2000); Associate Professor Waseda University, (2000-2014); Professor, Waseda University, (2014-); Visiting Associate Professor, Japan Aerospace Exploration Agency (2004-2007).